
Structural basis for iron piracy by pathogenic Neisseria.This conformation is predicted to greatly accelerate the cleavage reaction, and the other residues found to be involved are in agreement with experimental observations. The simulations reveal an unusual conformation of the catalytic asparagine residue, seen in less than 2% of all atomic structures, that is supported by the unique electrostatic environment created by neighboring residues. We have carried out simulations on the native pre-cleaved state as well as on a number of biochemically characterized mutants. After transport, the N-terminal domain is typically self-cleaved, freeing it from the membrane. For example, certain virulence factors are secreted through the use of so-called “auto-transporters”, proteins that export themselves! The auto-transporter consists of two domains, a membrane-bound barrel at the C-terminus and a secreted domain at the N-terminus. Just as bacteria need to import molecules across the outer membrane, they sometimes need to export them as well. Model of the full-length autotransporter EspP. In particular, we see in multiple contexts that the interior domain of the transporter unfolds, generating a pathway for the substrate across the membrane. We have proposed that energy is transduced through a mechanical coupling between the inner and outer membrane complexes, supported by simulations that demonstrate the strength of the TonB-transporter connection mediated by hydrogen bonds, as well as the response of the transporter to the application of force. The mechanisms of this interaction and how it causes transport are still open questions in the field of microbiology. To get energy for this task, they utilize an inner membrane protein, TonB, that couples across the periplasm to an outer membrane transporter for import of, e.g., vitamin B12 or iron extracted from human transferrin. Nonetheless, bacteria often need to actively import large and/or scarce nutrients across the outer membrane.

As such, energy cannot be generated or stored here.
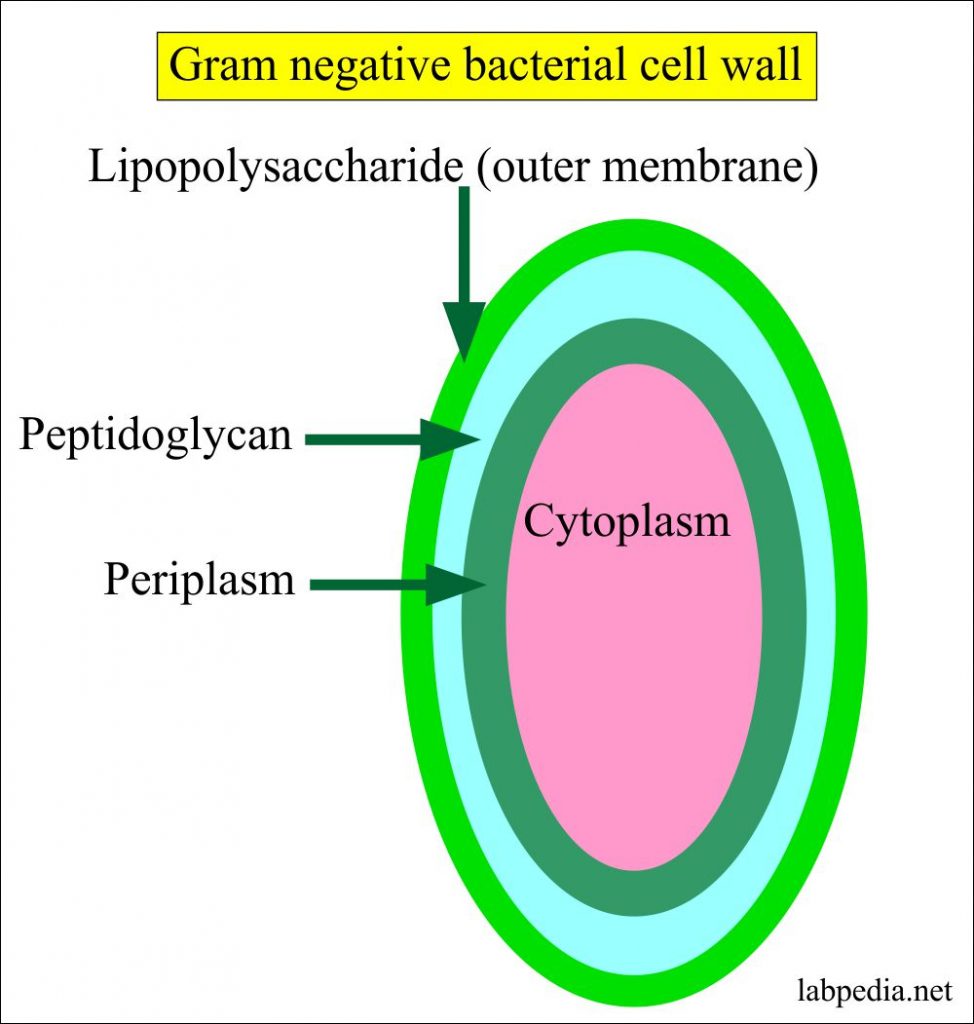
Cell wall of gram negative bacteria full#
TonB (red) “pulling” on the lumenal domain of the transporter BtuB (blue), the connection being mediated by hydrogen bonds (yellow).Īnother unique aspect of the outer membrane is that it’s full of porins, which permit the diffusion of small molecules relatively easily across it.


 0 kommentar(er)
0 kommentar(er)
